JURNAL TEKNOLOGI LABORATORIUM
Haruna Isiyaku Umar, Ijeoma Akunna Duru, Uchechi Emmanuela Enenebeaku, Lynda Chioma Ngozi-Olehi, Christian Ebere Enyoh, Chidi Edbert Duru
doi:10.29238/teknolabjournal.v11i1.344
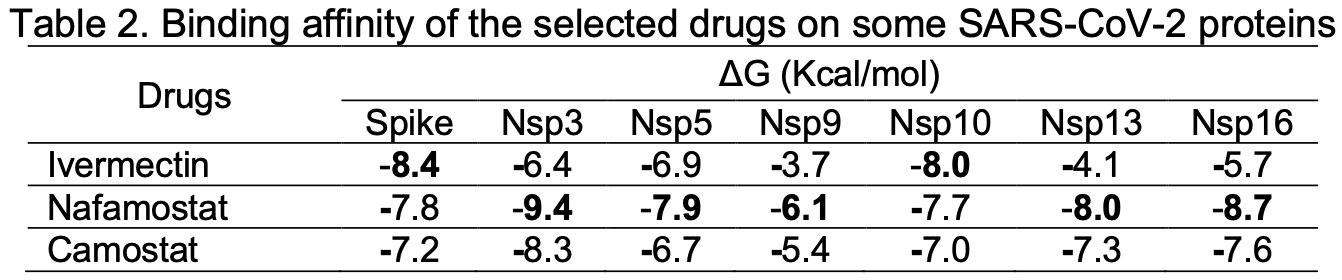
The search for potential oral drugs either through synthetic routes or by drug repurposing for combating the dreaded covid-19 virus is still ongoing. The coronavirus spike glycoprotein and several other non-structural proteins play crucial roles in the replication and transmission of this virus. Recent research have identified ivermectin, nafamostat, and camostat as promising drug inhibitors of SARS-CoV-2 target proteins. The broad-spectrum inhibitory action of ivermectin, nafamostat, and camostat on the spike glycoprotein and some non-structural proteins of this virus was studied in silico. The spike glycoprotein, nsp3, nsp5, nsp9, nsp10, nsp13, and nsp16 were selected for this study and were
AUTHORS' CONTRIBUTIONS CED: Conceptualization, Data curation, Supervision, Methodology, Software HIU: Conceptualization, Supervision, Methodology, Data curation, Software. IAD: Visualization, Investigation. UEE: Visualization, Investigation. LCN: Original draft preparation, Writing-Reviewing and Editing. CEE: Original draft preparation, Writing-Reviewing and Editing.
FUNDING INFORMATION No funds, grants, or other support was received.
DATA AVAILABILITY STATEMENT All data generated or analyzed during this study are included in this published article.
DISCLOSURE STATEMENT The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors. The data is the result of the author's research and has never been published in other journals. The authors declare that they have no competing interests.
References
Breining, Frølund, Højen, Camostatmesylate against SARS-CoV-2 and COVID-19-Rationale, dosing and safety, Basic Clin. PharmacolToxicol
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res
Chaccour, Casellas, Blanco-Di Matteo, Pineda, Fernandez-Montero et al., The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebocontrolled, randomized clinical trial, EClinicalMedicine,
doi:10.1016/j.eclinm.2020.100720Chen, Malone, Llewellyn, Grasso, Shelton et al., Structural basis for helicase-polymerase coupling in the SARS-CoV-2 replication-transcription complex, Cell,
doi:10.1016/j.cell.2020.07.033Decroly, Imbert, Coutard, Bouvet, Selisko et al., Corona virus nonstructural protein 16 is a Cap-0 binding enzyme possessing (Nucleoside-2'O)-methyltransferase, Activity J. Virol
Durdagi, Aksoydan, Dogan, Sahin, Shahraki, Sreening of clinically approved and investigation drugs as potential inhibitors of COVID-19 main protease: A virtual drug repurposing study,
doi:10.26434/chemrxiv.12032712.v1Duru, Duru, Adegboyega, In Silico identification of compounds from Nigella sativa seed oil as potential inhibitors of SARS-CoV-2 targets, Bulletin of the National Research Centre,
doi:10.1186/s42269-021-00517-xDuru, Duru, García, Enenebeaku, Computational modeling of the activity of metronidazole against EhGα1 of Entamoeba histolytica enhanced by its copper and zinc complexes, Chemistry Africa,
doi:10.1007/s42250-021-00245-9Duru, Haruna Umar, Duru, Enenbeaku, Ngozi-Olehi et al., Blocking the interactions between human ACE2 and coronavirus spike glycoprotein by selected drugs: a computational perspective, Environmental Analysis Health and Toxicology,
doi:10.5620/eaht.2021010Faheem, Kumar, Sekhar, Kunjiappan, Jamalis et al., Druggable targets of SARS-CoV-2 and treatment opportunities for COVID-19, Bioorg,
doi:10.1016/j.bioorg.2020.104269Hao, Wojdyla, Zhao, Han, Das et al., Crystal structure of middle East respiratory syndrome coronavirus helicase, PLoSPathog
Haruna, None, Jurnal Teknologi Laboratorium
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Jia, Yan, Ren, Wu, Wang et al., Delicate structural coordination of the severe acute respiratory syndrome coronavirus nsp13 upon ATP hydrolysis, Nucleic acids Research
Krafcikova, Silhan, Nencka, Boura, Structural analysis of the SARS-CoV-2 methyltransferase complex involved in RNA cap creation bound to Sinefungin, Nature Communications,
doi:10.1038/s41467-020-17495-9Ma, Wu, Shaw, Gao, Wang et al., Structural basis and functional analysis of the SARS-corona virus nsp14-nsp10 complex, ProcNatlAcad Sci
Ma-Lauer, Carbajo -Lozoya, Hein, Muller, Deng et al., Nsp3 down-regulates SARS corona virus replication and is targeted by the SARS-unique domain and PL pro via E3 ubiquitin ligase RCHY1, Proc. Natl. Acad. Sci
Macchiagodena, Pagliai, Procacci, Identification and potential binders of the main protease 3CL(pro) of the covid-19 via structurebased ligand design and molecular modeling, ChemPhysLett
Marcolino, Pimentel, Barão, What to expect from different drugs used in the treatment of COVID-19: A study on applications and in vivo and in vitro results, European journal of pharmacology,
doi:10.1016/j.ejphar.2020.173467Ramanathan, Robb, Chan, mRNA capping: Biological functions and applications, Nucleic acid Research
Rosas-Lemus, Minasov, Shuvalova, Inniss, Kiryukhina et al., High-resolution structures of the SARS-CoV-2 2′-O-methyltransferase reveal strategies for structure-based inhibitor design, Sci. Signal
Rosas-Lemus, Minasov, Shuvalova, Inniss, Kiryukhina et al., The crystal structure of nsp10-nsp16 heterodimer from SARS-Cov-2 in complex with S-adenosylmethionine,
doi:10.1101/2020.04.17.047498Sawicki, Sawicki, Younker, Meyer, Thiel et al., Functional and genetic analysis of coronavirus replicasetranscriptase proteins, PLoSPathog
Snijder, Decroly, Zierbuhr, The non structural proteins directing corona virus RNA synthesis and processing, Adv. Virus. Res
Stroganov, Novikov, Zeifman, Stroylov, Chilov, TSAR, a new graph-theoretical approach to computational modeling of protein sidechain flexibility: modeling of ionization properties of proteins, Proteins,
doi:10.1002/prot.23099Trott, Olson, AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading, J. Computational Chemistry
Umar, Josiah, Saliu, Jimoh, Ajayi et al., In-silico analysis of the inhibition of the SARS-CoV-2 main protease by some active compounds from select African plants, J TaibahUniv Medical Sci,
doi:10.1016/j.jtumed.2020.12.005Watanabe, Allen, Wrapp, Mclellan, Crispin, Sitespecific glycan analysis of the SARS-Cov-2 spike, Science
Wolff, Melia, Snijder, Barcena, Double membrane vessicles as platforms for viral relication, Trends in Microbiology,
doi:10.1016/j.tim.2020.05.009Yamamoto, Matsuyama, Li, Takeda, Kawaguchi et al., Identification of nafamostat as a potent inhibitor of Middle Eastrespiratory syndrome coronavirus S Protein-mediated membranefusion using the split-protein-based cell-cell fusion assay, Antimicrobial Agents and Chemotherapy
Zeng, Deng, Shi, Ye, Wang et al., Dimerization of coronavirus nsp9 with diverse modes enhances its nucleic acid binding affinity, J Virol
Zhangy, Li, Xia, Guo, Zhou, Structural basis for the recognition of SARS Cov-2 by full-lenght human ACE2, Science